Jeffrey N. Keller PhD, Rapid Analytics | E: [email protected]
How to Leverage the Success of MMCREA Moving Forward
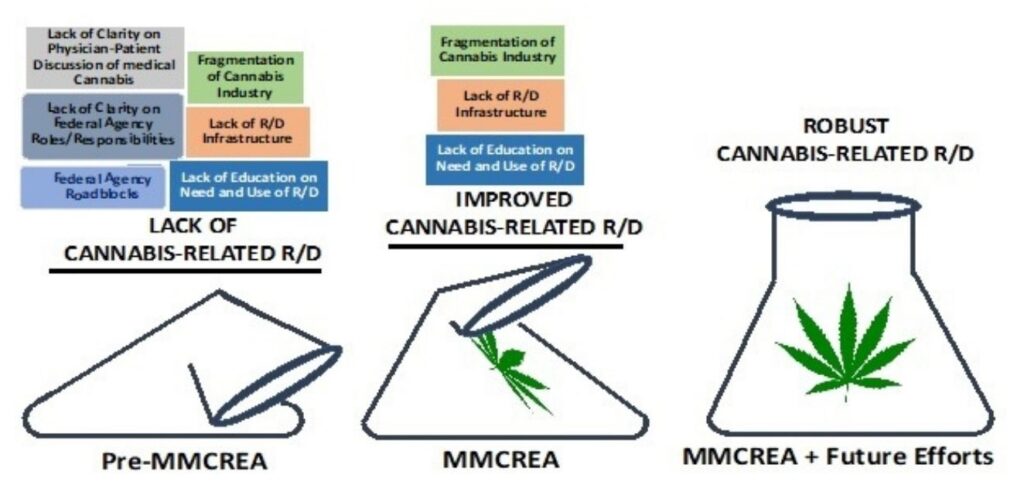
There are several direct and indirect impacts of the MMCREA that are likely to serve as a strong catalyst for both short-term and longer-term improvements in cannabis-related R/D. For example, the MMCREA is the first stand-alone bi-partisan legislation passed by both the House and Senate and therefore provides a roadmap for successful cannabis-related legislation in the future. The MMCREA also importantly provides much-needed Federal legitimacy to the topic of cannabis-related R/D and identifies some key components to target with future legislative, advocacy, and research investment efforts.
Allow Federal Tax Breaks for R/D Efforts
Internal Revenue Service (IRS) 280E currently prevents companies involved in the cannabis industry from taking advantage of several standard corporate tax breaks, including the routine deductions for R/D costs, and only allows for cannabis companies to deduct the cost of goods. Several States including California and New York allow, and other States are in the process of passing legislation to allow, cannabis businesses operating under select licenses to take State tax deductions that are not exempt Federally due to IRS 280E. Because MMCREA clearly provides Federal support for many aspects of cannabis-related R/D there is a unique opportunity in the coming years to remove or attenuate the restrictions imposed by IRS 280E on the cannabis industry, particularly as they relate to the topic of tax deductions for R/D expenses.
Reduce Fragmentation of Cannabis Industry
Fragmentation in the cannabis industry is a nearly unavoidable consequence of the fact there are 36 States and the District of Columbia that have varying degrees of legalization for cannabis use, ranging from restrictive medical cannabis use to legalization of recreational cannabis use. The fragmentation of the cannabis industry across various States has led to a lack of standardization and an inability to build key infrastructures that are required for a robust R/D environment. The provisions of the MMCREA around the requirement of Federal agencies to ensure an adequate supply of research-grade cannabis, and to report regularly to Congress on any obstacles to cannabis-related R/D provide a unique opportunity to begin reducing the fragmentation that currently plagues the cannabis industry. By focusing on multi-state, intrastate, and interstate processes and systems as part of the MMCREA it is likely that a systematic and continual decrease in the fragmentation of the cannabis industry can occur.
Common Standards, Terminology, and Procedures
Similar but distinct from the establishment of a common “language” for conducting cannabis-related R/D there is a need to establish core databases for each of the different forms of research including agronomy, biochemistry, pharmacology, drug/target development, pre-clinical research, and clinical research. By working from common databases for much, but not all, of cannabis-related research, the impact of research findings is compounded with each scientific effort. The move to common core databases is a common feature of most mature scientific pursuits and has paid off in both anticipated and unanticipated ways for the scientific community working in fields working in nearly every type of scientific research. The requirements of MMCREA provide a clear framework to initiate the development and growth of these core databases in the context of cannabis-related R/D.
Key Components in Addition to MMCREA that Are Needed
Peer-Review
Peer review is a central feature of any viable and longstanding R/D effort and is a key component of R/D that is notably absent for cannabis-related R/D efforts. While a smattering of peer-reviewed cannabis-related publications occurs annually in traditional scientific journals, these articles are not routinely read or cited by most individuals actively working in cannabis-related R/D efforts.
Although the basis for this reality is clearly multifactorial, it is likely the root cause is the current lack of a journal that focuses on the day-to-day interest of individuals actively focused on cannabis-related R/D. In particular, there is an urgent need for peer-reviewed research that provides data-driven policies and procedures for the agronomy, analytical testing, pharmacology, safety, and clinical testing aspects of the cannabis industry.
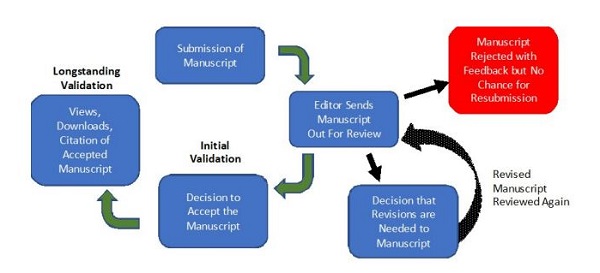
The conceptual framework for peer-review involves scientists/authors submitting appropriately designed and properly reported findings from their research to an established journal so that an editor and editorial board can have the manuscript reviewed anonymously by knowledgeable peers in the field (Figure 2). The outcome of peer-review produces a result of rejection, acceptance, or much more frequently a requirement for further revision and future review of the revised manuscript (Figure 2). This first step of peer-review results in the production of manuscripts that have results that have been initially validated by the review from knowledgeable peers in the field (Figure 2). The additional and longstanding validation of the manuscript and its findings comes from the number of times the work is viewed, downloaded, and ultimately cited by peers working in the field (Figure 2). In a relatively young field like cannabis R/D there is also an important role for the peer-review process to educate scientists and authors about the scientific method, basic aspects of statistical analysis, and the utilization of commonly accepted methods and procedures.
There are multiple types of journal formats that can be utilized for the publication of accepted manuscripts, but ultimately these journals fall into either reader-sponsored journals (requiring readers to have a paid subscription or pay for access to each individual article) or author-sponsored journals (where authors pay handling and processing fees to allow the articles to be freely available for viewing by readers).
The author-supported model of publishing is commonly referred to as open-access, and open-access has taken off in popularity in recent years with the requirement from the National Institutes of Health (NIH) and other funding agencies to make the research findings derived from their funding be freely available to the scientific community. Developing open-access journals for cannabis-related R/D efforts could allow for the accelerated development of a robust and much-needed peer-review solution for the field. In particular, the development of open-access journals that build off the momentum of the annual Emerald Conference and MJ Biz conferences could be particularly helpful in jumpstarting cannabis-related R/D efforts.
Funding of Cannabis-Related R/D
The ultimate accelerator to cannabis-related research will be the establishment and growth of funding mechanisms to support research in the field. Funding from the National Institutes of Health, US Department of Agriculture, and other Federal agencies will need to be complemented by fundings from private corporations and advocacy groups supporting cannabis-related research. The MMCREA provides the framework for Federal, private, and not-for-profit entities to begin meaningful efforts to provide research funding in the most critical areas of cannabis-related research.
Education on R/D
While nearly everyone involved in the cannabis field states the need for more research the majority of individuals and organizations do not have the plan to pursue or incorporate research into their mission and/or business model. In the past, the slow pace and obstacle-ridden pursuit of cannabis-related research promoted a lack of education on the importance and need for research in most cannabis-focused organizations. The MMCREA provides a key inflection point in the evolution of cannabis-related research, but without a corresponding effort to educate the cannabis community on the importance of incorporating research and research-based data into their core mission and business model, it is likely that cannabis-related research will remain in a suppressed state. It is crucial to the long-term vibrancy of cannabis-related research to continually bring the various for-profit, not-for-profit, and advocacy stakeholders together to help shape the future of cannabis-related research to ensure the maximal impact of MMCREA and related efforts.
References
- Lowe H, Toyang N, Steele B, Bryant J, Ngwa W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int J Mol Sci. 2021;22(17):9472. Published 2021 Aug 31. doi:10.3390/ijms22179472
- Bilbao A, Spanagel R. Medical cannabinoids: a pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med. 2022;20(1):259. Published 2022 Aug 19. doi:10.1186/s12916-022-02459-1
- Kaur S, Sharma N, Roy A. Role of Cannabinoids in Various Diseases: A Review. Curr Pharm Biotechnol. 2022;23(11):1346-1358. doi:10.2174/1389201023666211223164656
Editors’ Note: This is an excerpt from our Monthly Playbook. If you would like to read the full monthly playbook and join the thousands of others you can sign up below.